Wednesday, September 7, 2011
Tuesday, September 6, 2011
Bhopal disaster
Bhopal disaster
for more details please visit:- http://www.gurukpo.com/

Methyl isocyanate is also manufactured from N-methylformamide and air. In the latter process, it is immediately consumed in a closed-loop process to make methomyl.[9] Other manufacturing methods have been reported

Saturday, September 3, 2011
OPTICAL ISOMERISM
Chiral and achiral molecules
The essential difference between the two examples we've looked at lies in the symmetry of the molecules.
If there are two groups the same attached to the central carbon atom, the molecule has a plane of symmetry. If you imagine slicing through the molecule, the left-hand side is an exact reflection of the right-hand side.
Where there are four groups attached, there is no symmetry anywhere in the molecule.
 A molecule which has no plane of symmetry is described as chiral. The carbon atom with the four different groups attached which causes this lack of symmetry is described as a chiral centre or as an asymmetric carbon atom.
A molecule which has no plane of symmetry is described as chiral. The carbon atom with the four different groups attached which causes this lack of symmetry is described as a chiral centre or as an asymmetric carbon atom.
The molecule on the left above (with a plane of symmetry) is described as achiral.
Only chiral molecules have optical isomers.
The relationship between the enantiomers
One of the enantiomers is simply a non-superimposable mirror image of the other one.
In other words, if one isomer looked in a mirror, what it would see is the other one. The two isomers (the original one and its mirror image) have a different spatial arrangement, and so can't be superimposed on each other.
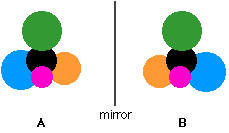 If an achiral molecule (one with a plane of symmetry) looked in a mirror, you would always find that by rotating the image in space, you could make the two look identical. It would be possible to superimpose the original molecule and its mirror image.
If an achiral molecule (one with a plane of symmetry) looked in a mirror, you would always find that by rotating the image in space, you could make the two look identical. It would be possible to superimpose the original molecule and its mirror image.
The essential difference between the two examples we've looked at lies in the symmetry of the molecules.
If there are two groups the same attached to the central carbon atom, the molecule has a plane of symmetry. If you imagine slicing through the molecule, the left-hand side is an exact reflection of the right-hand side.
Where there are four groups attached, there is no symmetry anywhere in the molecule.
The molecule on the left above (with a plane of symmetry) is described as achiral.
Only chiral molecules have optical isomers.
The relationship between the enantiomers
One of the enantiomers is simply a non-superimposable mirror image of the other one.
In other words, if one isomer looked in a mirror, what it would see is the other one. The two isomers (the original one and its mirror image) have a different spatial arrangement, and so can't be superimposed on each other.
For more details please visit http://www.gurukpo.com/
GEOMETRICAL ISOMERISM
| Geometric (cis / trans) isomerismHow geometric isomers arise These isomers occur where you have restricted rotation somewhere in a molecule. At an introductory level in organic chemistry, examples usually just involve the carbon-carbon double bond - and that's what this page will concentrate on. Think about what happens in molecules where there is unrestricted rotation about carbon bonds - in other words where the carbon-carbon bonds are all single. The next diagram shows two possible configurations of 1,2-dichloroethane. If you draw a structural formula instead of using models, you have to bear in mind the possibility of this free rotation about single bonds. You must accept that these two structures represent the same molecule: | |
Subscribe to:
Comments (Atom)

